
EPA Prepares for SBREFA Panel on Ethylene Oxide
The U.S. Environmental Protection Agency (EPA) has announced that it may convene a Small Business Advocacy Review panel under the Small Business Regulatory Enforcement and Fairness Act (SBREFA) in anticipation of new regulations on emissions of ethylene oxide (EtO) from commercial sterilization and fumigation operations.
The Office of Advocacy encourages small businesses that would be directly affected and their representatives to participate in the SBREFA panel process and bring their knowledge and experience to this rulemaking.
The purpose of the SBREFA panel is to gather advice and recommendations from small businesses likely to be directly regulated by an EPA rule before a proposed rule is published. Small Entity Representatives (SERs) are asked about the likely costs and benefits of regulatory action and possible regulatory alternatives and flexibilities. Then, the panel, which includes the Chief Counsel for Advocacy, the Administrator of the Office of Information and Regulatory Affairs, and program office staff from EPA, writes a report based on this feedback that becomes part of the record supporting the proposed rule.
EPA has begun gathering information that will support this rulemaking, publishing an Advance Notice of Proposed Rulemaking, issuing a request for data from a limited number of affected firms, and posting a request for SER nominations for participation in the SBREFA panel consultations. These pieces would come together with the convening of the panel and consultation with SERs for the EtO commercial sterilizers, expected in early 2020.
EtO Sterilizers
Ethylene Oxide is one of the most abundantly produced gases worldwide. Most is used to produce other products, like PVC, synthetic fibers and other plastics. It is also a very effective sterilizer. It can be used to sterilize everything from entire pallets of medical devices packed for shipping to individual tools in a hospital surgery. EtO is used in place of other sterilization methods because it is suitable for items that are sensitive to heat, moisture or radiation. Manufacturers prefer EtO because it can penetrate packaging materials, which allows them to sterilize their products right before shipment to wholesaler and retail distributors.
EPA recognized EtO as a Hazardous Air Pollutant (HAP) under the Clean Air Act in 1990 and set EtO emission standards for commercial sterilization operations in 1994. More recent scientific work has raised significant questions about whether those standards are sufficiently protective of human health. EPA classified EtO as a human carcinogen in December 2016 and published new estimates of the risk cause by EtO exposure. When EPA issued its National Air Toxics Assessment (NATA) in 2018 (reflecting 2014 emissions data), the report used this higher estimate and showed more populated areas at risk. Representatives of the chemical industry have challenged the EPA’s conclusions, but EPA has not yet responded. Nonetheless, EPA is moving forward with the periodic “technology review” required under the Clean Air Act and indicates that emission standards need to be tightened in response to this new information.
One major issue that will complicate new standards will be the importance of EtO to the medical device supply chain. The first problem is that EtO is an integral part of a medical device approval. When a manufacturer requests approval for a medical device from the Food and Drug Administration (FDA), it includes details about the manufacturing process, including the particulars of sterilization. Manufacturers cannot simply substitute one sterilization method for another without going through another FDA review process. The second problem is that EtO sterilization dominates the industry. The current estimate is that fifty percent of all sterile medical devices in the U.S. are sterilized with EtO. FDA is working with industry to develop alternatives to EtO, but there are no existing direct substitutes. There are some small-scale alternatives, which require manufacturers to re-design their production and distributions processes, but there is currently not enough capacity to replace EtO entirely. For these reasons, any EPA action that reduces the availability of EtO sterilization could have a significant effect on the availability of a range of medical devices. EPA will need to work with FDA to consider the consequences of its regulatory actions on the medical device supply chain.
EPA Actions
In preparation for a proposed rule, EPA published an Advance Notice of Proposed Rulemaking (ANPRM) on Dec. 12, 2019. This ANPRM asks questions about the use of EtO by commercial sterilizers, including available EtO emissions data and possible emission control technologies. It also asks about the small businesses in the industry and how they may differ from larger businesses. Comments on the ANPRM are due on Feb. 10, 2020.
EPA has concurrently sent a request for information directly to a selection of businesses in the industry. This request is for data about individual facilities. Although only directed to nine businesses, EPA hopes to get enough information to develop reasonable cost and benefit estimates of its proposal. For more information about this data request, see EPA’s webpage on this rulemaking and search for “section 114.”
Because this source category is already regulated, EPA has been able to identify 27 small businesses (out of a total 59 businesses) that would be directly regulated by a change to the existing emission standards. For this rule, small businesses are defined by the SBA size standards for the industries shown below.
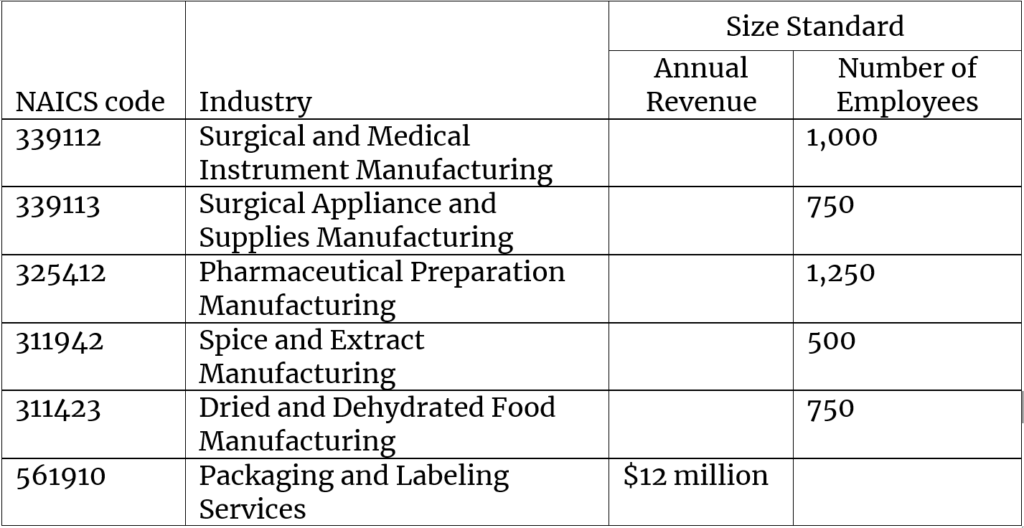
Any significant tightening of the emission standards would likely have a significant economic impact on a substantial number of small entities. In anticipation of this possibility, EPA is preparing for a SBREFA panel. It has posted information about the possible panel on its website and requested nominations. The Office of Advocacy welcomes nominations and self-nominations even after the posted deadline of December 20, 2019.
For more information about this rulemaking, the SBREFA panel process or to nominate a small business for the SBREFA panel consultations, contact Assistant Chief Counsel Dave Rostker.
Dave Rostker is an Assistant Chief Counsel for Advocacy whose portfolio includes Clean Air Act regulations. He can be reached at david.rostker@sba.gov or by phone at 202-205-6966.
